![SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale](https://cdn.numerade.com/ask_images/789c0aaf514c4932b19dafca225933f9.jpg)
SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale
![Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_599e6db7e641a.png)
Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
2. All atoms, except the | Download Scientific Diagram Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig5/AS:667921089052683@1536256205687/Representation-of-the-unit-cell-of-NiH2O6NO32-All-atoms-except-the.jpg)
Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram
![Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+(aq)is blue in colour, give reason - YouTube Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+(aq)is blue in colour, give reason - YouTube](https://i.ytimg.com/vi/pONeuFEpHnU/maxresdefault.jpg)
Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+(aq)is blue in colour, give reason - YouTube
![SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d](https://cdn.numerade.com/ask_previews/543ac424-a25c-42c6-88c8-ae8f1d1a07d2_large.jpg)

![a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram](https://www.researchgate.net/profile/Petya-Petkova-7/publication/279288804/figure/fig1/AS:1132476902711296@1647014942438/a-Absorption-spectrum-of-the-NiH2O6-complex-in-the-spectral-region-395-795-nm_Q320.jpg)


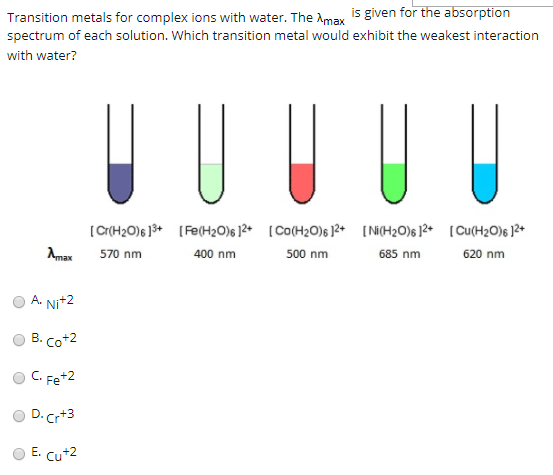

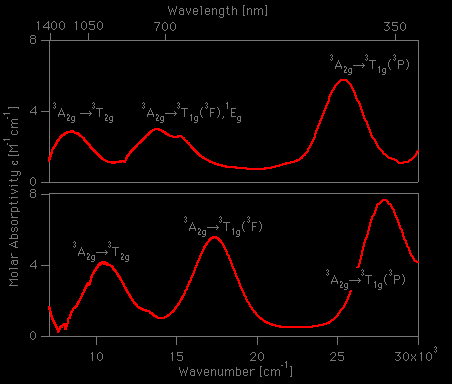
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)
![A solution of [Ni(H2O)6]^(+2) is green but a solution of [Ni(CN)4]^(-2) is colourless explain. A solution of [Ni(H2O)6]^(+2) is green but a solution of [Ni(CN)4]^(-2) is colourless explain.](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/647809885_web.png)

2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022024814007805-gr3.jpg)